Time-related Complications in Patients with Traumatic Spinal Cord Injury according to the AIS Impairment Scale Grade: A Retrospective Review
Article information
Abstract
Purpose
The aim of this study was to analyze time-dependent complications in patients with traumatic spinal cord injury (SCI) as a function of their initial neurological status determined by the ASIA Impairment Scale (AIS) grade.
Methods
Consecutive patients with SCI who visited the emergency department at Asan Medical Center from 2005 to 2019 were included, and their electronic medical records were analyzed according to four time periods from initial visit to hospital discharge. Time-dependent SCI-related complications, including medical, urinary, musculoskeletal, and neurological complications and pain, were analyzed as well as the association between SCI complications and AIS grade. In-hospital recovery from neurological damage and longterm outcomes were also evaluated.
Results
Of the 632 SCI patients who visited the emergency department during the study period, 110 patients were included in the study. The complication rates in patients initially assessed as AIS grades A, B, C, D, and E were 53.6%, 16.4%, 14.6%, 13.6%, and 1.8%, respectively. The most common complications after SCI were UTIs (n=37; 33.6%), pressure ulcers (n=36; 32.7%), neuropathic pain (n=34; 30.9%), and pneumonia (n=33; 30.0%). Patients with a poor neurological status (AIS grade A) had more medical complications and lower neurological resilience than did those with better neurological status (AIS grades B-E).
Conclusion
Post-SCI complications differed by time after SCI and neurological status. Nurses should be aware that complications can occur at any time in patients with traumatic SCI. Patients with poorer AIS grades should be particularly closely observed and receive preventive care for complications.
INTRODUCTION
Traumatic spinal cord injury (SCI) is defined as an injury resulting from an insult inflicted on the spinal cord that compromises neural functions, regardless of the completeness of neural injury [1]. SCI can affect several other biological systems (e.g., urinary, cutaneous, respiratory, cardiovascular, and musculoskeletal systems) and induce secondary impairments, which in turn confer a negative impact on social participation and quality of life [2]. Inhospital complications after SCI exacerbate the complexity of patient care and may prolong hospital stay, as well as result in treatment failure, increased risk of additional complications, and significant economic and social costs [3]. The mortality of SCI patients who survive their initial traumatic injury is not primarily due to spinal cord damage itself but rather due to later complications [4]. Accurate prediction and adequate management of complications after SCI are important for the prevention of longterm secondary impairments [5]. It is very important to review all aspects of the care process in order to have a more holistic view of the needs of patients with SCI, and learning to manage patients, including complications, is essential to improving patients' quality of life [6]. Because quickly recognizing a patient's signs and symptoms and providing prompt treatment can save a patient's life, nurses must be able to identify when they are needed and provide appropriate support, including preventative measures [7]. Therefore, effective management of patients with SCI is to strengthen and support the identification of complications and the exchange of information between doctors, nurses, caregivers and patients to make the management process easier for all involved [6,8].
More than 50.0% of patients diagnosed with an acute traumatic SCI experience at least one in-hospital complication [2,5,9]. In Chevroletto's study, 41.6% of hospitalization complications occurred, and American Spinal Injury Association impairment scale (AIS) grade A group developed 4.5 times higher than AIS B, C, and D groups, and immobility are a major determinant of complications and require special nurse treatment [3]. Systematic nursing based on the nurse's comprehensive understanding of the patient's condition can improve the nursing effect, reduce the incidence of complications, and effectively alleviate the patient's psychological pressure, thereby improving the patient's standard of living and helping to promote clinical practice [9,10]. Consequently, assessments of time-dependent SCI complications may help properly identify and manage these complications in high-risk patients, resulting in better outcomes.
Although recent advances in the acute care of patients with SCI have reduced the occurrence of SCI-related complications, less is known about the time-dependent characteristics of complications. In this study, we systematically assessed the time-dependent complications from injury to rehabilitation in SCI patients.
METHODS
1. Research Design
This study was a retrospective review of electronic medical records.
2. Participants and Data Collection
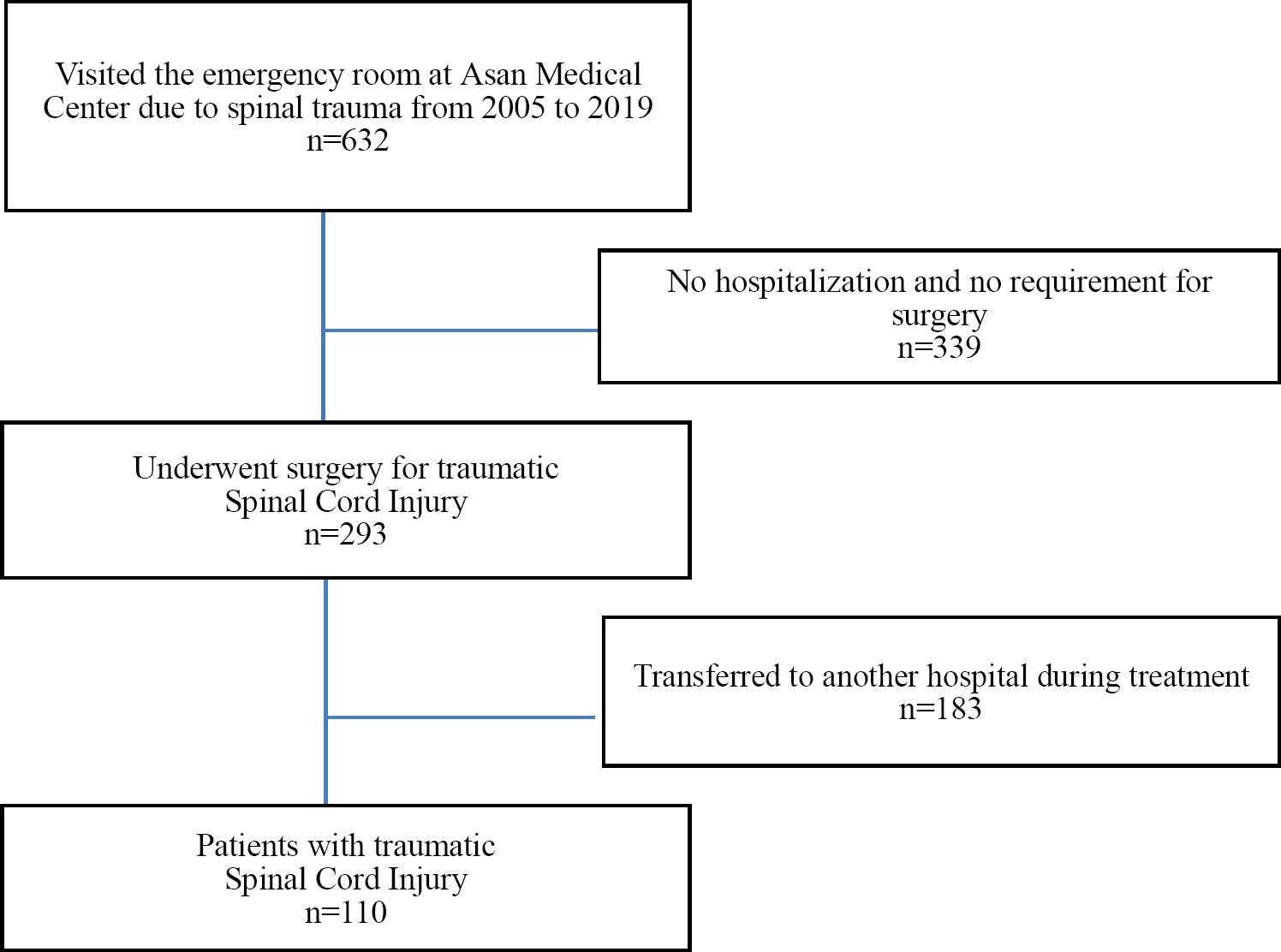
We identified consecutive patients with SCI who visited the emergency room at Asan Medical Center (Seoul, Re-public of Korea) from 2005 to 2019. We included adult patients (age >18 years) who underwent spinal surgery for traumatic SCI, followed by acute care in a neurosurgical intensive care unit (ICU) and rehabilitation by a specialist. A total of 632 patients visited the emergency room due to spinal trauma, and 293 patients underwent surgery, excluding 339 patients who did not require hospitalization or surgery. Excluding 183 patients who were transferred to other hospitals after surgery, 110 patients who received rehabilitation treatment were included in the study (Figure 1).
The electronic medical records of all patients were analyzed, and complications were assessed in four time periods from admission to the emergency room to hospital discharge: period 1 (time in the emergency room and pre-operative admission to the ICU), period 2 (immediate postoperative ICU stay), period 3 (postoperative stay in the neurosurgery ward), and period 4 (stay in the comprehensive rehabilitation ward).
The primary aims of this study were to investigate the time-dependent SCI-related complications, including medical (e.g., pressure ulcers, pneumonia, atelectasis), urinary (e.g., autonomic dysreflexia, neurogenic bladder, urinary tract infection [UTI]), musculoskeletal, and neurological complications (e.g., pain). In addition, the associations between SCI complications and neurological status, as determined by AIS grade, were evaluated in addition to in-hospital recovery from neurological damage and long- term outcomes.
3. Measurements
We analyzed the following clinical information from the electronic medical record: gender, total cause of injury, level of injury, combined organ injuries, time to arrival at the emergency room after trauma, time to surgery, length of ICU stay, length of postoperative general ward stay, length of rehabilitation treatment stay, total length of in hospital and AIS during the four time periods. Results of neurological examinations (e.g., motor, sensory, bulboca-vernosus reflex, and other examinations) were divided according to the AIS grades and recorded in electronic records, with all results reviewed by three independent neu-rosurgeons and a clinical nurse specialist. Physical examinations were standardized by the American Spinal Injury Association using the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) [11]. Complications were grouped according to their system-atic classifications, such as cardiovascular, respiratory, musculoskeletal, genitourinary, digestive, skin, and neurological complications. In addition, the results of radio-logic examinations, referral consult information, and labo-ratory tests were analyzed.
4. Ethical Consideration
The study was approved by the Institutional Review Board of Asan Medical Center (approval No. 2020-1422). The study was conducted in accordance with the Declaration of Helsinki.
5. Data Analysis
The collected data were analyzed using IBM SPSS Sta-tistics for Windows, version 21 (IBM Corp., Armonk, NY, USA), and percentage, frequencies, means, standard devi-ation (SD) of the mean, minimum, maximum, median, and interquartile range (IQR) were calculated and analyzed.
RESULTS
1. Participants' General Characteristics
A total of 110 patients were included in this study. Their demographics and clinical characteristics are shown in Table 1. Eighty-Seven subjects were male (79.1%), and the mean age was 43.0 years (SD=1.5). The causes of SCI were falls (36.4%), traffic accidents (34.5%), and sports trauma (18.2%), in order, and the levels of injury were cervical (57.3%), thoracic (25.4%), and lumbar (17.3%) spine. Sixty- one (55.5%) patients had other organ injuries, 39 (35.5%) bone fractures, 36 (32.7%) lung injuries, and 18 (16.4%) had brain injuries. The average time taken to visit the emergency room after trauma was 19.8 hours, the average waiting time until surgery was 4.9 days. The median ICU stay was 5 days (IQR 3~8, min~max, 2~46), the median neurosurgery ward stay was 12 days (IQR 7~22, min~max, 5~63), the median rehabilitation ward stay was 28.5 days (IQR 24~43, min~max, 5~113), and the median total length of stay in hospital was 48.5 days (IQR 39~76, min~max, 18~163). Clean intermittent catheters were used by 50.0% of patients for urination, with 19.1% having indwelling catheters at discharge.
2. Neurological Improvement: Changes in the AIS Grade after SCI
The initial AIS grades were A in 59 (53.6%) of patients, B in 18 (16.4%), C in 16 (14.6%), D in 15 (13.6%), and E in 2(1.8%). At the time of discharge, AIS grades were A in 45(40.9%), B in 18 (16.4%), C in 15 (13.6%), D in 26 (23.6%), and E in 6 (5.5%) (Figure 2).
3. Incidence of Complications as a Function of AIS Grade
The complication rates in patients initially assessed as AIS grades A, B, C, D, and E were 53.6%, 16.4%, 14.6%, 13.6%, and 1.8%, respectively. The number of complications during hospitalization in patients with SCI was 25.5% in the order of two cases, 23.6% in the order of three cases, and 17.3% in the order of one complication (Table 2).
The most common complications after SCI were UTIs 37(33.6%), pressure ulcers 36 (32.7%), neuropathic pain 34(30.9%), and pneumonia 33 (30.0%). UTIs occurred from day 8 to day 73 (median, 34 days), pressure ulcers from day 2 to day 80 (median, 33.5 days), neuropathic pain from day 3 to day 83 (median, 27.0 days), and pneumonia from day 1 to day 100 (median, 12 days) (Figure 3).
The overall complication rates during period 1 (preop-erative), period 2 (immediate), period 3 (postoperative), and period 4 (rehabilitation) were 9.0%, 3.1%, 30.6%, and 57.3%, respectively. Pneumonia was the most common complication in the early stage of SCI, occurring in 7(2.7%) at the time of admission to the emergency room, 3(1.2%) during ICU stay, 18 (7.1%) in the postoperative ward, and 5 (2.0%) in the rehabilitation ward. Complications occurred the most at 57.3% during the rehabilitation period after spinal cord injury, and the frequency of occurrence was high in the order of UTI 12.5%, neuropathic pain 10.6%, pressure ulcer 8.6%, and orthostatic hypotension 5.9% (Table 3).
DISCUSSION
We performed this study to assess time-dependent complications after traumatic SCI as a function of initial AIS grade. By identifying factors that are predictive of post- SCI complications, this study may contribute to the devel-opment of complication management guidelines for traumatic SCI.
The demographic characteristics of the study population, including age, sex distribution, causes and levels of injury, and comorbidities, were similar to those of previous studies [3,5,11]. The initial AIS grades in the current study population were A in 53.6% of patients, B in 16.4%, C in 14.6%, D in 13.6%, and E in 1.8%. These grades were worse than in other study populations [5,11]; for example, a study of SCI patients at eight rehabilitation centers in the Netherlands reported that 18.9%, 16.2%, 15.3%, 47.7%, and 1.8% had an initial AIS grade of A, B, C, D, and E, respectively [11]. Another study of patients at nine hospitals reported that 40.0%, 16.0%, 15.0%, and 29.0% of patients had an initial AIS grade of A, B, C, and D, respectively [5]. Thus, compared with these previous studies, the present study included more severely injured patients.
We assessed the pattern of neurological recovery as a function of the initial AIS grade, and at the time of discharge, it decreased by 12.7% in the AIS grade A group, increased by 10.0% in the AIS grade D group, and 3.7% in E group. Similarly, Badhiwala et al (2021) found that the initial neurological condition decreased from 49.6% to 37.9% in AIS grade A and improved from 21.2% to 33.2% in AIS grade D over time, and that the neurological outcome de-pended on the initial neurological status [12]. Another study reported that the severity of the AIS grade was associated with the power of predictors for the occurrence of complications, and more intensive management of the prevention of complications was needed [5]. In this study, the complication rate of patients in the AIS grade A group in the initial stage was 53.6%, which was higher than that of other groups, and the number of complications was also high. Jentzsch et al. (2021) suggested that patients with poorer clinical symptoms (i.e., AIS grade) are more likely to have worse clinical outcomes even after 1 year, so patients with poorer clinical symptoms should receive treatment for complications early [13]. These results indicate that meticulous care is needed for longterm complications beyond perioperative care. Nurses should be knowledge-able about the prognosis of patients with spinal cord injury and educate and manage patients and their families, considering that the continuous management of complications in the future will affect the patient's quality of life.
We found that pulmonary complications were the most common complications early period after SCI. The most common complication in prospective study of SCI patients was pulmonary complications (34.0%) [14]. Similarly, patients with a poor neurological grade showed a lower rate of neurological recovery and a higher rate of mortality, which are likely due to an increase in cardiopulmonary complications [3,14]. Rouanet et al. (2017) reported that pulmonary complications are a major cause of morbidity and mortality in the acute phase of SCI, with incidence rates ranging from 36.0% to 83.0% [15]. SCI patients with apnea at the time of injury either die before going to the hospital, more than 50.0% of people with cervical spine injuries are intubated or may require ventilation support between 12 hours and 6 days after injury due to spinal shock and spinal cord edema [16]. Decreased lung capacity, se-cretion retention, and autonomic dysfunction all play a role, leading to complications such as atelectasis, pneumonia, or respiratory failure requiring mechanical ventilation [15]. Artificial airway care such as ventilator and trache-otomy is important in the early stages, and continuous chest physical therapy and respiratory rehabilitation training to prevent atelectasis and promote respiratory clear-ance are effective in preventing respiratory complications such as pneumonia [17]. Respiratory complications can occur from acute to chronic stages, and patients with upper cervical injuries particularly require meticulous nursing care for the respiratory system.
The most common complications after the acute phase of SCI were immobility-related complications such as UTI and pressure ulcers, while other late complications included neuropathic pain, orthostatic hypotension, and ileus.
In our present study, the incidence of UTI was the most frequent complication at 33.6%, and occurred between 8 to 73 days after SCI. The incidence of ileus was 11.8%, and and the onset period was from 6 to 76 days, indicating that excretory dysfunction continued to occur from the acute stage of SCI. We found that 50.0% of patients required clean intermittent catheterization, and that 19.1% required a semi-permanent indwelling catheter at discharge. Excretory dysfunction after SCI causes a variety of bladder and bowel problems, including unexpected incontinence and complicated self-care protocols (e.g., intermittent catheterization), threatening the patient's health, as well as inter-fering with social activities and daily life, seriously affecting quality of life [18]. In this study, the incidence of UTI in patients with SCI was 33.6%, which was not significantly different from the 31.7% reported in South Korean study in patients with traumatic and non-traumatic SCIs [19]. The risk of UTI is significantly higher with the severity of SCI (e.g., AIS A, B classifications) [19,20], so it is necessary for nurses to evaluate the AIS injury classification in order to provide individualized care for the prevention and management of UTI [19]. Education on self-management methods such as aseptic voiding techniques, and hand hy-giene, prevention of bladder distension, the balance between fluid intake and urinary output, and cyclical emptying can go a long way in reducing the burden and anxiety of bladder and bowel management [18,19]. Depending on the patient's neurological damage and excretory dysfunction, timely identification and active and individualized nursing management are required.
Spinal cord injury causes motor paralysis and loss of sensation, and the risk of pressure ulcers is high because you have to lie down for a relatively long time [10,21]. In our study, pressure sores occurred in 32.7%. Another study found that 37.5% of patients admitted to acute hospitalization and inpatient rehabilitation occurred at least one pressure ulcer [21]. Pneumonia and higher injury severity (AIS A grade) were significantly associated with the incidence of pressure ulcers [21]. Preventive management of pressure sores requires preventive care for pressure injuries, including adequate support surfaces, wheelchairs and seating systems, frequent repositioning, optimal mo-bility techniques, nutrition, physical activity, and weight management [22].
Pain following SCI is common and often chronic, About 53.0% (ranging from 34.0% to 74.0%) of Americans with a SCI have neuropathic pain [23]. Of the patients in the present study, 30.9% experienced neuropathic pain, which occurred 3 to 83 days after SCI. Chronic pain after SCI is a common secondary complication, with neuropathic pain cited as one of the most distressing and debilitating conditions, leading to poor quality of life, depression, and sleep disturbances [24]. Principles of neuropathic pain management recommend self-management strategies for reducing pain intensity, coping, and improving function. Consider pharmacological and non-pharmacological treatments or multidisciplinary management to control activity, mood, and sleep. A pain management program including pain management education, cognitive behav-ioral therapy, and exercise is recommended [25]. Nurses need support so that they can take an consider in pain management and intervene actively at an early stage.
Through this study, it was confirmed that various complications occurred over time in SCI patients. This contrib-utes to a comprehensive understanding of the ongoing patient condition in the intensive care unit, postoperative general care and rehabilitation management. It also has the advantage of contributing to the accumulation of evidence for the management and treatment of spinal cord injury complications. Reasonable and effective nursing care is required to improve the recovery and living standards of patients with SCI. This study has several limitations. First, this research institute is a tertiary general hospital, not a trauma center, and there is a possibility that the study population was concealed because there were no beds for trauma patients. Second, it is possible that ex-treme values of arrival time to the emergency room are included because it includes cases where emergency room visits are delayed, such as patients in unstable conditions who cannot be transported immediately after trauma. Therefore, the possibility of generalization to SCI patients is limited.
CONCLUSION
Managing complications of SCI patients is a process and outcome of nursing, and nurses play an important role in the clinical environment. Our study investigated the complications that occur during treatment in SCI patients and will contribute to the accumulation of related data. Nurses must be aware of potential complications in order to reduce or prevent secondary injury and damage in SCI patients. Nurses should carefully monitor changes in factors associated with complications during the treatment planning process and modify and apply personalized nursing practices based on research-based evidence.
Notes
CONFLICTS OF INTEREST
The authors declared no conflict of interest.
AUTHORSHIP
Study conception and design acquisition - Kim J & Shin YS; Data collection - Kim J; Data analysis & Interpretation - Kim J & Shin YS; Drafting & Revision of the manuscript - Kim J & Shin YS.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.